Tag: FDA
-

FDA rejects ANH petition to roll back suppression of nutrient-disease health claims
[ad_1] As reported by NutraIngredients, ANH filed the petition in September 2025 asking FDA to allow 118 nutrient–illness statements drawn straight from authorities publications and public-facing supplies produced by scientists on the Nationwide Institutes of Well being (NIH), the Facilities for Illness Management and Prevention (CDC) and different federal well being companies. FDA denied 116…
-

From state battles to tariff relief and FDA fights, NPA delivered wins in 2025
[ad_1] That’s not a slogan or hyperbole. It’s a file of motion that stretches from the earliest battles over client entry to pure well being merchandise to passage of the Dietary Complement Well being and Schooling Act of 1994 (DSHEA), consequential regulatory actions and landmark litigation. NPA’s legacy issues as a result of in 2025,…
-
FDA seizes $1 million in 7-OH products
[ad_1] The seizure, which was valued at roughly $1 million, centered on meals and dietary complement merchandise containing concentrated 7-OH as an added ingredient. Earlier this summer season, the FDA beneficial that 7-OH-containing merchandise be scheduled below the Managed Substances Act as a result of the compound is more and more acknowledged as having potential…
-

Asa Waldstein on FDA, FTC and NAD enforcement trends
[ad_1] Regulatory scrutiny from the Federal Commerce Fee (FTC), Nationwide Promoting Division (NAD) and the Meals and Drug Administration (FDA) has gained momentum in 2025 throughout CPG classes, together with dietary and dietary dietary supplements. For instance, as beforehand reported by NutraIngredients, corporations like Olly and Ryze Superfoods have been beneath the microscope earlier this…
-

ANH pushes FDA to broaden access to medical foods
[ad_1] The non-profit group used the discharge of its Strategic Roadmap and Action Plan to advocate for adjustments to the Meals and Drug Administration (FDA)’s laws that restrict entry to medical meals. Particularly, ANH takes challenge with the restriction of medical meals to the remedy of uncommon ailments beneath the Orphan Drug Act. Alongside this…
-

FDA warns about synthetic kratom, Amway’s new probiotic products, Active Parenting
[ad_1] Final week’s large information included the U.S. FDA sending warning letters about merchandise containing 7-hydroxymitragynine (7-OH), Amway’s HEM Pharma partnership for brand new probiotic merchandise, and the rise of ‘energetic parenting’. The U.S. FDA despatched seven warning letters to corporations that it says are illegally advertising and marketing merchandise containing 7-hydroxymitragynine (7-OH), also referred…
-

Jones leaves FDA, remembering AHPA’s Michael McGuffin, Balchem’s FC Bayern Munich partnership, and Nestlé’s infant formula growth
[ad_1] This week’s huge information throughout the worldwide vitamin and dietary supplements industries contains the abrupt resignation of Jim Jones as head of the U.S. Meals and Drug Administration’s Human Meals Program, Balchem’s partnership with the ladies’s FC Bayern Munich, HMOs serving to Nestlé rebound China toddler method gross sales, and remembering AHPA’s Michael McGuffin.…
-

Can Changes in FDA Regulation Affect Your Beauty Products?
[ad_1] The world wellness economy is valued at 1.8 trillion {dollars} yearly, with bodily exercise, magnificence and private care, and dietary consuming taking the highest spots. However, proper now, it’s extremely debatable how nicely we actually are—and what the phrase “wellness” even means. In case you ask Robert F. Kennedy Jr., Individuals are extremely unhealthy—and…
-

FDA Recalls Antidepressant Duloxetine Bottles With This Lot Number
[ad_1] The U.S. Meals and Drug Administration has recalled greater than 7,000 bottles of duloxetine—an antidepressant bought underneath the model title Cymbalta that’s typically prescribed to deal with persistent ache. The Class II recall was initiated because of the remedy containing ranges of N-nitroso-duloxetine, a chemical that may be poisonous if consumed at sure ranges.…
-
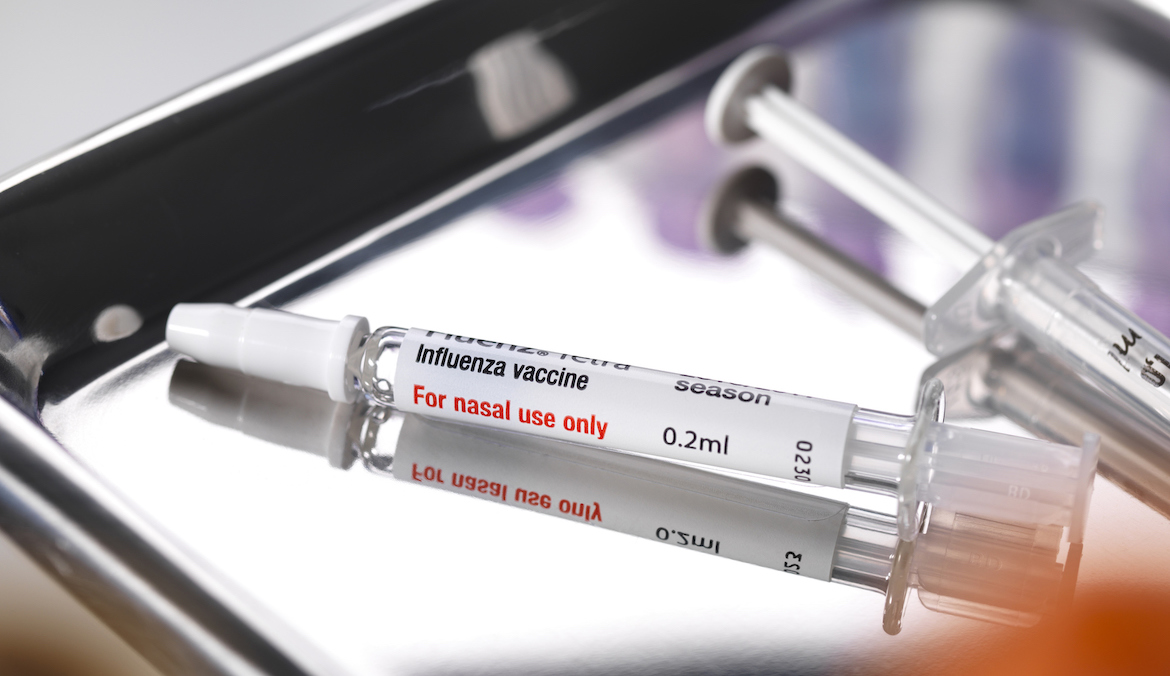
FDA Approves Nasal Spray Flu Vaccine for At-Home Use
[ad_1] The first-ever needleless flu vaccine that may be given at dwelling was simply authorised by the U.S. Meals and Drug Administration. FluMist is a nasal spray that gives safety in opposition to influenza A and B, and it’s now authorised for self-administration in adults as much as age 49 and caregiver-administration for teenagers ages…