[ad_1]
Well being claims are one of the vital efficient ways in which dietary complement firms can convey the advantages of their merchandise. Nevertheless, there are strict legal guidelines relating to well being claims, and widespread confusion round how and when these legal guidelines come into play.
Writing within the journal Nutraceuticals, Spanish meals regulatory specialists described the regulation of dietary dietary supplements as “one of the vital advanced subjects in meals legislation,” with well being claims cited as one of the vital misunderstood areas.
Well being claims have been unregulated in Europe till 2007 when the European Fee launched a authorized framework referred to as the Well being and Vitamin Claims Regulation. The regulation governs the usage of vitamin and well being claims on meals merchandise, together with dietary dietary supplements, bought throughout the European Union.
The first objective of this piece of laws is to make sure that any claims made a few meals’s dietary or well being advantages are truthful and never deceptive to shoppers. However what’s the distinction between a nutrient declare and a well being declare?
Nutrient claims vs well being claims
Kristy Coleman, authorized director at UK legislation agency Ashfords and an AFN certified nutritionist, mentioned well being claims are one of the vital frequent queries dropped at her by shoppers, typically accompanied by important confusion in regards to the variations between well being claims, nutrient claims and medical claims.
“A well being declare is any declare that states, suggests or implies {that a} relationship exists between a meals, or one among its constituents, and well being,” she advised NutraIngredients. “This consists of any reference to a operate of the physique, psychological efficiency or physiological impact.”
For instance, “Vitamin C contributes to the traditional operate of the immune system” is a well being declare, as is “Vitamin D contributes to the upkeep of regular bones and tooth”.
The wording is purposeful, utilizing language that’s clear and correct, and doesn’t exaggerate the potential advantages of the ingredient. The context through which the declare is used additionally issues, Coleman added, in addition to any imagery, graphics and different artistic property.
Vitamin claims, alternatively, relate solely to the composition of the product, equivalent to ‘low sugar’, ‘supply of calcium’ or ‘excessive in fiber’.
“A vitamin declare is any assertion that means a meals or complement has explicit helpful dietary properties because of the vitality it supplies, supplies at a diminished or elevated price, or doesn’t present, or because of the vitamins or different substances it incorporates or doesn’t comprise,” Coleman mentioned.
There are additionally sure thresholds in place for nutrient claims. For instance, within the EU, a complement or meals merchandise should comprise at the very least 6 grams of fiber per 100 grams for the declare ‘excessive fiber’ for use.
In distinction, a medical declare is any declare {that a} product can deal with, forestall or remedy a illness, together with each bodily and psychological well being. These claims are reserved solely for pharmaceutical medicine or drugs and can’t be used for dietary dietary supplements.
Forms of claims
What’s a well being declare?
Any assertion a few relationship between meals and well being.
What’s a vitamin declare?
Any assertion relating solely to the composition of a meals product.
What’s a medical declare?
Any assertion {that a} product can deal with, forestall or remedy a illness. These claims can’t be used for dietary dietary supplements.
Which well being claims can be utilized? And what are the roles of EFSA and the European Fee?
Within the EU, vitamin and well being claims can be utilized provided that they’re licensed and seem within the annex to the Regulation and on the EU Register of Well being Claims, which lists all permitted vitamin claims and all licensed and non-authorized well being claims.
European nations which don’t belong to the European Union have their very own well being declare registers. For instance, British firms can use claims that seem on the Nice Britain Vitamin and Well being Claims Register, if the product meets the circumstances of use.
The EU Register was devised by the European Fee, which reviewed a whole bunch of submitted claims by Member States earlier than drawing up a constructive checklist of established claims. The finalized first model of the checklist was printed in 2012.
Any claims added to this checklist post-2012 have principally been submitted by firms in search of a well being declare for one among their very own elements. Every declare is examined by EFSA after which reviewed by the European Fee and Member States.
“EFSA is a scientific advisory physique,” meals regulation knowledgeable Luca Bucchini advised NI. “It assesses—amongst different issues—well being declare functions and new knowledge and supplies a scientific opinion to the Fee. EFSA doesn’t approve or reject—it solely supplies a scientific opinion. Nevertheless, most often, the Fee follows EFSA’s recommendation.”
The European Fee, alternatively, proposes authorized acts to Parliament and to the council.
“All minor authorized acts, such because the checklist of licensed claims, accredited novel meals and meals components, are made by the Fee,” Bucchini mentioned.
“All acts of the Fee are accredited by the specialists of the Member States by majority. This implies new well being claims can solely be accredited if nearly all of the Member States are on board. The selections are of a technical nature, so objections are usually not political. Some Member States might, for instance, want extra warnings, or completely different circumstances of use.”
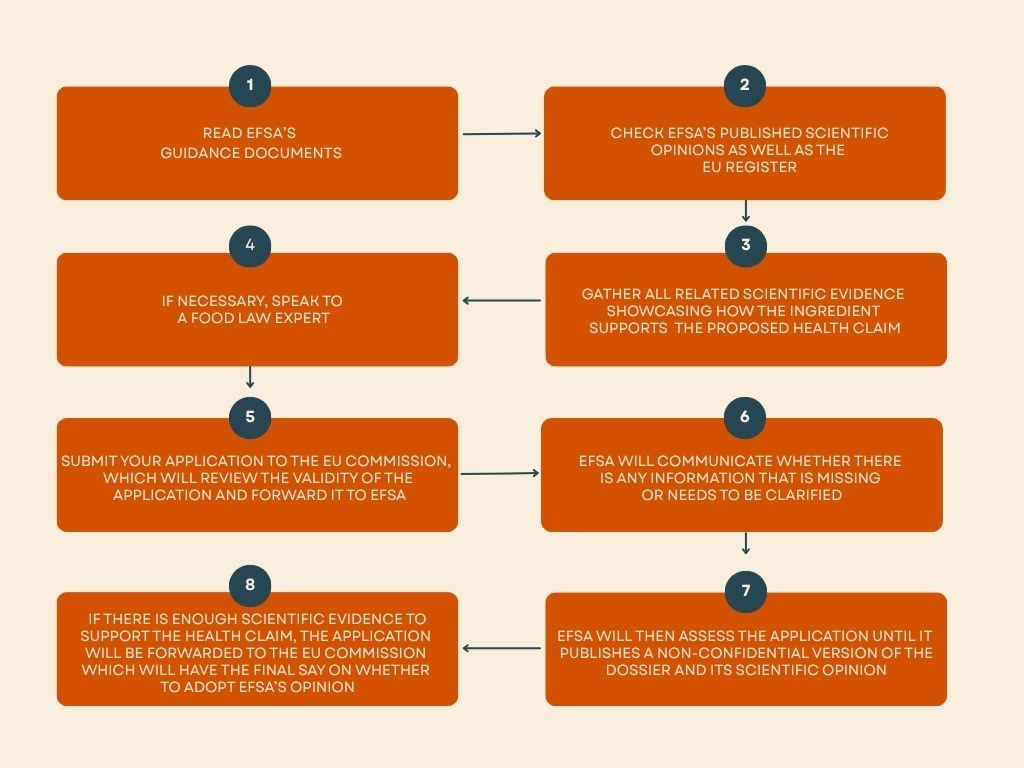
Methods to apply for a brand new well being declare
Acquiring a brand new well being declare is tough, with EFSA estimating that round 70% of evaluated claims have been rejected because of an absence of scientific proof. As of 2023, solely round 260 well being claims had been accredited to be used within the EU by the European Fee.
However, ingredient firms can apply for a brand new well being declare by submitting an utility to EFSA. The kind of utility required depends upon the kind of declare the applicant is making.
Put merely, firms making use of for purposeful claims will apply underneath Article 13 of Regulation (EC) No 1924/2006, whereas functions for illness danger discount or kids’s growth will apply underneath Article 14.
There’s a slight differentiation underneath Article 13, with 13(1) well being claims supported by usually accepted scientific proof. For instance, “Calcium contributes to regular muscle operate.” In distinction, 13(5) well being claims relate to newly developed scientific analysis and/or safety of proprietary knowledge. For instance, “Tomato extract helps keep regular platelet aggregation, which contributes to wholesome blood stream.”
Typically, most purposeful well being declare functions will now fall underneath Article 13(5) given that the majority well being claims supported by usually accepted scientific proof have already been accredited.
Provexis’ tomato focus, marketed as Fruitflow, grew to become the primary product in Europe to acquire an accredited well being declare underneath Article 13(5) in 2009, with solely a handful of 13(5) claims accredited since.
“Article 13 claims are speculated to be typical and never deceptive as a result of they don’t seem to be associated to any particular illness,” Katia Merten-Lentz, companion at Meals Science & Regulation Companions advised NI. “Importantly, most of them have been used throughout Europe far earlier than the adoption of the Regulation.”
“Article 14 claims are thought of by the European Fee as probably the most harmful for shoppers,” she added. “They’re associated to a discount of illness danger and may solely be made the place they’ve been licensed in accordance with the process laid down in Articles 15 to 18, which is the strictest process of the regulation.”
For instance, an instance of an Article 13(5) declare can be: “Inexperienced kiwifruit contributed to the upkeep of regular defecation” or “Sugar beet fiber contributes to a rise in fecal bulk”.
Comparatively, “Calcium and vitamin D cut back the danger of osteoporotic fractures” is a illness danger discount declare and would fall underneath Article 14, as would “Elevated maternal folate consumption reduces the danger of neural tube defects.”
You will need to word the distinction between Article 14 well being claims and medical claims. Whereas medical claims, that are prohibited for meals dietary supplements, declare {that a} product can deal with, forestall or remedy a illness, firms can search a declare {that a} product might be able to cut back illness danger underneath Article 14. Nevertheless, that is probably the most tough kind of declare to acquire.
Forms of well being claims
Article 13(1)
Refers to purposeful well being claims supported by usually accepted scientific proof
o Inexperienced kiwifruit contributes to the upkeep of regular defecation
o Sugar beet fibre contributes to a rise in fecal bulk
Article 13(5)
Refers to purposeful well being claims supported by newly developed scientific analysis and/or safety of proprietary knowledge
o Tomato extract helps keep regular platelet aggregation, which contributes to wholesome blood stream
o Owing to its caffeine content material, black tea improves consideration
Article 14
Refers to illness danger discount well being claims
o Calcium and vitamin D cut back the danger of osteoporotic fractures
o Growing maternal folate standing reduces the danger of neural tube defects
Use of well being claims
The one well being claims that can be utilized on the packaging of a product are these that are accredited and seem on the EU register. Coleman mentioned there’s “very restricted flexibility” relating to well being claims, highlighting that the legislation applies not solely to specific statements but in addition to implied or oblique claims.
“Which means claims equivalent to ‘really feel your finest’, ‘restore stability’ or ‘help your life-style’ should still be captured as well being claims, relying on context,” she mentioned. “If such phrases are used along with references to physiological results or vitamins recognized to have a well being operate, they’re more likely to be thought of implied well being claims.”
The precise wording of the well being declare itself can be an vital facet to contemplate, in keeping with Merten-Lentz.
“The nationwide authorities have a really strict method and think about that the wording adopted by the European Fee should be exactly adopted,” she mentioned. “Nevertheless, some nationwide steerage—equivalent to a Belgium’s and the UK’s—present some helpful examples of ‘tolerance’ relating to the best way a licensed well being declare may very well be barely rephrased.”
Implying an unapproved well being declare through imagery can be prohibited. It’s because the European Fee defines a declare very broadly as “Any message or illustration, which isn’t necessary underneath Group or nationwide laws, together with pictorial, graphic or symbolic illustration, in any type, which states, suggests or implies {that a} meals has explicit traits.”
For instance, merchandise that use a picture of the intestine alongside a product geared toward intestine well being, notably if positioned alongside references to vitamins or well-being language, would represent a well being declare.
“That is typically neglected,” Merten-Lenz mentioned. “It is vitally tempting to contemplate {that a} image can be much less legally dangerous than a full message on a packaging, however this isn’t the case.”
Total, Coleman highlights the significance of remembering that regulators assess your entire presentation of the product, that means that the wording, imagery and branding will all be assessed.
“If, when taken as an entire, the mixture of images and textual content implies a well being profit, it could be handled as a well being declare,” she mentioned. “If not licensed, such a declare can be illegal.”
What are the dangers of non-compliance?
Meals legislation specialists say one of the vital frequent pitfalls for complement manufacturers and ingredient suppliers is assuming they’ll make a well being declare based mostly on outcomes from a scientific trial that helps an ingredient’s potential well being profit.
Even when there’s a wealth of scientific analysis to again up a selected well being declare, if it’s not on the EU Vitamin and Well being Claims Register, it can’t be used.
“Scientific research will not be utilized in client dealing with supplies to justify unauthorized claims,” Coleman mentioned.
“Even factual references to research can represent implied well being claims in the event that they recommend the product has a selected well being profit. It’s attainable to supply scientific materials to healthcare professionals in a non-promotional context, however entry to this content material should be appropriately restricted. The place such materials is utilized in client contexts, even with citations, it’s more likely to breach the principles until the underlying well being declare is permitted.”
Failing to adjust to these guidelines can lead to important penalties, together with hefty fines and requests to take away total product strains from the market.
“It’s clearly said within the Basic Meals Regulation Regulation 178/2002 that any meals positioned on the European market should be secure and compliant with all of the legal guidelines in pressure,” Merten-Lentz mentioned.
“Consequently, it signifies that in case a nationwide authority of management challenges a declare contemplating it in breach of Regulation 1924/2006, they’ll formally ask the meals enterprise operator to withdraw the non-compliant merchandise nonetheless in the marketplace and to assessment the packaging of those in inventory earlier than putting them of the market. That is on prime of fines for non-compliance.”
The severity of those fines differ from one Member State to a different. Exterior of the EU, the UK’s Competitors and Markets Authority (CMA) has just lately been given new powers to challenge fines of as much as £300,000, or 10% of turnover, for non-compliance.
“There are additionally reputational dangers and business penalties,” Coleman mentioned. “Merchandise could also be delisted by retailers, faraway from on-line platforms, or the model might face damaging publicity.”
[ad_2]
Source link

Leave a Reply